This rate law works for all values of a ≠ b. Ln[a] = −kt + ln[a] 0 [latex]frac{1}{[a]} = kt+(frac{1}{[a]_0})[/latex] plot needed for linear fit of rate data [a] vs.
Integrated Rate Law Second Order Derivation. The integrated rate expression is used to calculate the rate constant of the reaction. This rate law works for all values of a ≠ b.
 Using Graphs to Determine Rate Laws, Rate Constants, and From saylordotorg.github.io
Using Graphs to Determine Rate Laws, Rate Constants, and From saylordotorg.github.io
However, it avoids negative numbers if b > a. A second order rate law means that the rate of reaction depends on the concentration of two reactants raised to the first reaction, or one reaction raised to the second power. Complete step by step answer:
Using Graphs to Determine Rate Laws, Rate Constants, and
In mathematical language, these are first order differential equations because they contain the first derivative and no higher derivatives. This rate law works for all values of a ≠ b. These are inherently differential equations, because the rate is always defined as a change in concentration with time; Second order reactions ( j=2) the differential form of the rate law is:
 Source: slideshare.net
Source: slideshare.net
However, it avoids negative numbers if b > a. Complete step by step answer: => ln [a]0 [ a] 0 = c. So we have one over the concentration of [a]t minus one over the initial concentration of a, and then on the right side, this of course would just be kt. By elementary integration of these differential equations integrated.
 Source: youtube.com
Source: youtube.com
Rate = k[a] rate = k[a] 2: 𝑅 p =− [𝑨] 𝒕 = [𝑨] we separate the variables and integrate over the interval: 2nd order reaction integrated rate law 1 [𝐴𝐴] = 𝑘𝑘 +𝑑𝑑1 [𝐴𝐴]0 at time t = t1/2 [𝐴𝐴] = 1 2 [𝐴𝐴]0 therefore 1 [𝐴𝐴] = 2 ∙1 [𝐴𝐴]0 substitute for [a] 2 ∙1 [𝐴𝐴0] = 𝑘𝑘𝑑𝑑1⁄2+.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
A chemist calls them second order rate laws because the rate is proportional to the product of two concentrations. −d[r] dt = k[r]2 − d [ r] d t = k [ r] 2. These are inherently differential equations, because the rate is always defined as a change in concentration with time; Considering the scenario where one second order reactant.
 Source: slideshare.net
Source: slideshare.net
These are inherently differential equations, because the rate is always defined as a change in concentration with time; We can arrange this to get the integrated rate law: 𝑅 p =− [𝑨] 𝒕 = [𝑨] we separate the variables and integrate over the interval: −d[r] dt = k[r]2 − d [ r] d t = k [ r] 2. (2).
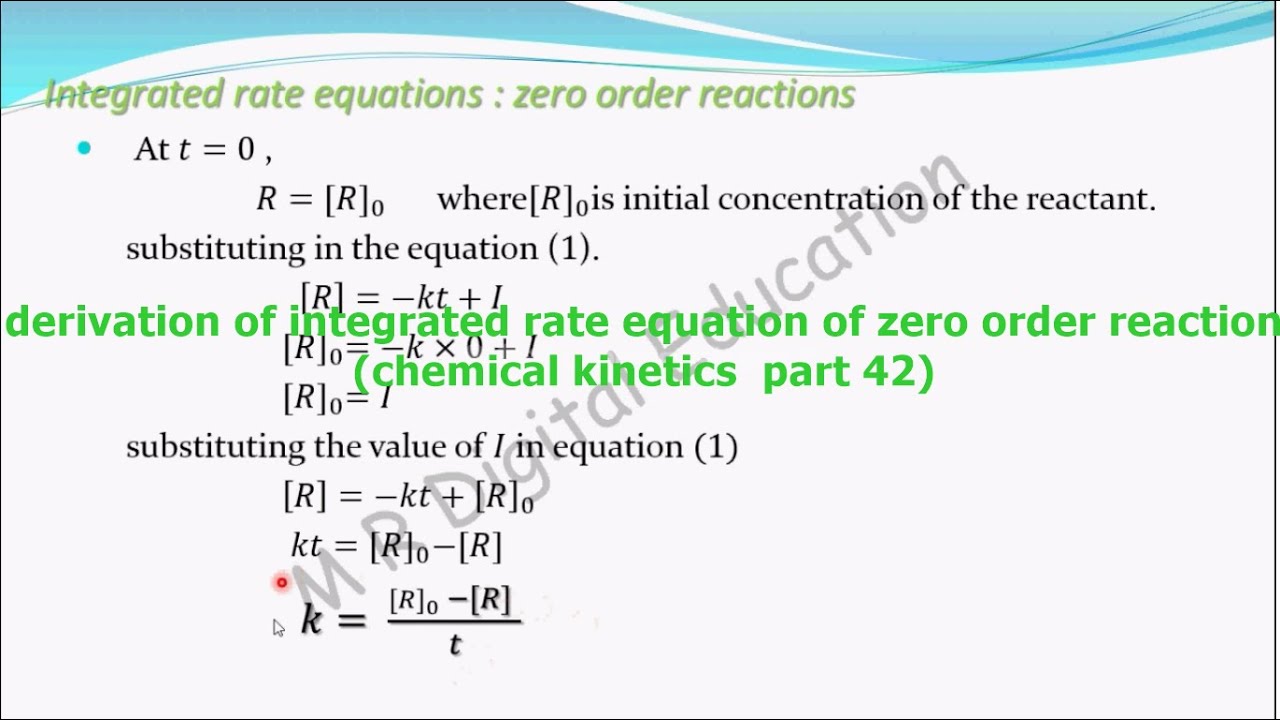 Source: youtube.com
Source: youtube.com
So this is our integrated rate law. Given that for a reaction of nth order the integrated rate equation is k 1 t n c 0 where and are concentration reactant at time initially respectively 3 4. Considering the scenario where one second order reactant forms a given product in a chemical reaction, the differential rate law equation can be.
 Source: tessshebaylo.com
Source: tessshebaylo.com
Bonus derive the integrated rate law for the second order rate equation v=k [a] [b] where the rate orders are ma = 1 and mb = 1. If a reaction is second order, a plot of _____ vs. Derive an integrated rate equation for constant the first order reaction tessshlo law you 4 laws derivation viziscience interactive labs chemical kinetics.






