Y is equal is to mx plus b. Ln[a]t = (−k)(t)+ln[a]0 y = mx+b ln [ a] t = ( − k) ( t) + ln [ a] 0 y.
Integrated Rate Law For Zero Order Reaction. It explains how to use the integrated rate laws for a zero order, first. So here�s the integrated rate law for a zero order reaction.
 From venturebeat.com
From venturebeat.com
It explains how to use the integrated rate laws for a zero order, first. The integrated rate law can be rearranged to a standard linear equation format: The common integrated rate laws.
Because this equation has the form y = mx + b, a plot of the concentration of a as a function of time yields a straight line. How long does it take? Because this equation has the form y = mx + b, a plot of the concentration of a as a function of time yields a straight line. Y is equal is to mx plus b.
 Source: venturebeat.com
Source: venturebeat.com
Rate = k[a]0 = k. On the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific amount of time (t) has passed; The integrated rate law can be rearranged to a standard linear equation format: Let us consider a general.
 Source: youtube.com
Source: youtube.com
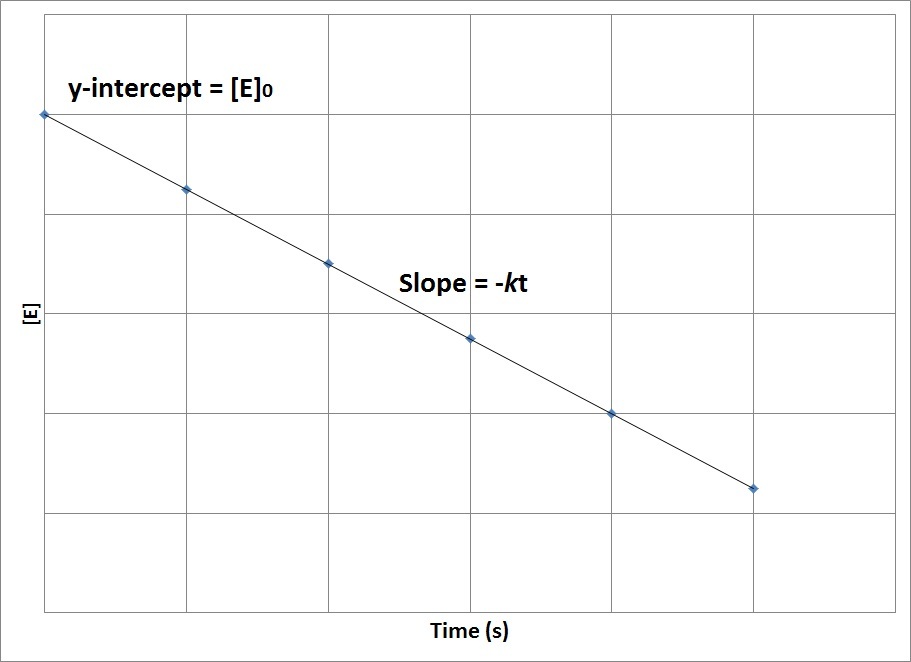
If we graph time on the x axis, on the y axis we have the concentration of a. Y is equal is to mx plus b. The common integrated rate laws. This chemistry video tutorial provides a basic introduction into chemical kinetics. [ a] t = − k t + [ a] 0 y = m x + b.
 Source: slideshare.net
Source: slideshare.net
So we would put time on the x axis. Rate = k[a]0 = k. They are used to determine the rate constant and the reaction order from experimental data. Ln[a]t = (−k)(t)+ln[a]0 y = mx+b ln [ a] t = ( − k) ( t) + ln [ a] 0 y = m x + b. Equation 1.6.2 has the.
 Source: slideserve.com
Source: slideserve.com
Ln[ ]=− g p+ln[ ]0 2a products or a + b products (when [a] = [b]) , rate = k[a] 2 the integrated rate law is 1/[a] = kt + 1/[a o] top. Where [a]0 is the initial concentration of reactant a. [ a] t = − k t + [ a] 0 y = m x + b. About.
 Source: opentextbc.ca
Source: opentextbc.ca
Ln[ ]=− g p+ln[ ]0 [a] = [a]0 − kt. Where [a]0 is the initial concentration of reactant a. [ a] t = − k t + [ a] 0 y = m x + b. Equation 1.6.2 has the form of the algebraic equation for a straight line, y = mx + b, with y = [a], mx =.
 Source: slideshare.net
Source: slideshare.net
It explains how to use the integrated rate laws for a zero order, first. Where [a]0 is the initial concentration of reactant a. So we would put time on the x axis. The common integrated rate laws. [ a] t = − k t + [ a] 0 y = m x + b.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
They are used to determine the rate constant and the reaction order from experimental data. 2a products or a + b products (when [a] = [b]) , rate = k[a] 2 the integrated rate law is 1/[a] = kt + 1/[a o] top. So here�s the integrated rate law for a zero order reaction. Where [a]0 is the initial concentration.






