For example, when sodium hydroxide reacts with hydrochloric acid (hcl), the hydroxide ion from naoh reacts with the hydrogen ion in hcl to form water (h2o). When hcl reacts with.
Hydrochloric Acid And Sodium Hydroxide Reaction Type. Naoh reacts with acid to produce a water and an ionic compound. Now as per the law of conservation of mass, mass is neither created nor destroyed in a chemical reaction.
 12 types of chemical reactions From slideshare.net
12 types of chemical reactions From slideshare.net
Is the reaction between sodium hydroxide and hydrochloric acid endothermic or exothermic? What is the difference between hydrochloric acid and hydrogen chloride? Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat.
12 types of chemical reactions
Naoh reacts with acid to produce a water and an ionic compound. The reaction between sodium hydroxide (naoh) and hydrochloric acid (hcl) is a neutralization reaction which results in the formation of a salt, sodium chloride (nacl) , and water (h2o). It is an exothermic reaction. There is a very simple, but very effective, way of measuring the time taken for a small fixed amount of precipitate to form.
 Source: edu.glogster.com
Source: edu.glogster.com
Naoh reacts with acid to produce a water and an ionic compound. This is called a neutralization reaction. In a double displacement reaction, the anions of the reactants switch the cations they are associated with. Hoh is h2o) how about a little understanding at no extra charge. Simply so, what is the word equation for hydrochloric acid and potassium hydroxide?
 Source: slideshare.net
Source: slideshare.net
The given reaction is as follows: Simply so, what is the word equation for hydrochloric acid and potassium hydroxide? Sodium hydroxide + hydrochloric acid → sodium chloride + water naoh + hcl → nacl + hoh (note: Sodium carbonate is the disodium salt of. Hydrochloric acid + aqueous sodium hydroxide 7.
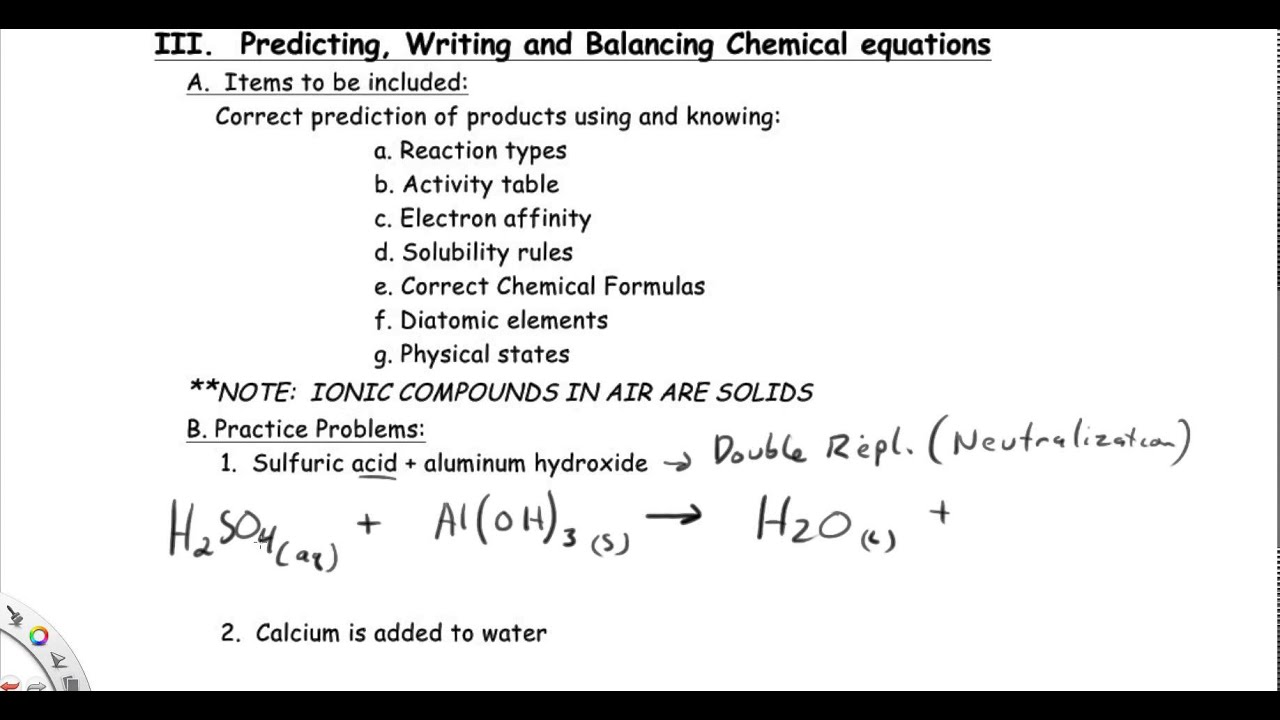 Source: youtube.com
Source: youtube.com
This double replacement makes this a double replacement reaction. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. And its equation reads as follows: We investigated the best remediation strategies to maximize arsenic removal efficiency for both soi. There is a very simple, but very effective, way of measuring the time taken for a small.
 Source: slideshare.net
Source: slideshare.net
The given reaction is as follows: Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Hcl + naoh = nacl + h₂o + q. Preparation and standardization of 0.1 m sodium. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat.
 Source: revise.im
Source: revise.im
This is called a neutralization reaction. Now as per the law of conservation of mass, mass is neither created nor destroyed in a chemical reaction. Sodium chloride is also known as common salt. This video shows what happens when an acid and a base are made to react. Is the reaction between sodium hydroxide and hydrochloric acid endothermic or exothermic?
 Source: slideshare.net
Source: slideshare.net
Hydrochloric acid(hcl) reacts with sodium hydroxide ( naoh) to form a colourless aqueous solution of sodium chloride ( nacl) salt. And its equation reads as follows: Sodium chloride is also known as common salt. In a similar way, sulphuric acid (h2so4) is neutralised by sodium hydroxide. We investigated the best remediation strategies to maximize arsenic removal efficiency for both soi.
 Source: slideshare.net
Source: slideshare.net
When dissolved in water, sodium carbonate forms carbonic acid and sodium hydroxide. The reaction in which hydrochloric acid (hcl) reacts with sodium hydroxide (naoh) to produce water (h2o) and sodium chloride (nacl) is a special type of double displacement reaction called a neutralization reaction. The end products are salt and water. The “reaction of sodium hydroxide and hydrochloric acid” the.
 Source: slideshare.net
Source: slideshare.net
The reaction between sodium hydroxide (naoh) and hydrochloric acid (hcl) is a neutralization reaction which results in the formation of a salt, sodium chloride (nacl) , and water (h2o). As the reacting acid is hydrochloric acid, then the salt produced will be a chloride. Naoh reacts with acid to produce a water and an ionic compound. There is a very.






